
In 2016, the USP[2] Chapter[1] “<1207> Package integrity evaluation – sterile products” was updated, seriously questioning the sole use of historical methods such as blue tests or microbiological tests. In this Chapter, the microbiological method is called “Microbial Challenge, Immersion Exposure” and the blue method is included in the so-called “Tracer Liquid” methods. These two methods are categorised as “probabilistic methods”. Probabilistic methods are characterised by the random nature of the phenomena that allow the liquid to pass through the samples. In contrast, the USP defines “deterministic methods” as physico-chemical techniques that enable quantifiable, reproducible results to be obtained with clearly defined and predictable detection limits.
In both cases, the microbiological or blue method, the basic principle is similar. The aim is to detect the passage of a liquid inside a container. The passage of the liquid is detected by staining or microbial growth.
Here we will look at the methylene blue method.
Methylene blue test method or Dye Ingress
The samples are first filled with product. An empty sample can also be tested, but generally the passage is favoured between two liquids. The samples are then immersed in a dye solution.
To improve the penetration of the dye, the samples are subjected to a vacuum for a set period of time, the product is then allowed to return to atmospheric pressure and the penetration time is allowed to elapse. This is the most important stage of the test.
Various dyes can be used, historically methylene blue solution (most often solutions dosed at 1g/L). At the end of the complete test cycle, the samples are cleaned and then read. Samples can be read visually or using a spectrophotometer. If any of the samples show internal coloration, the sample is declared a leak.
Positive controls and detection limits
Alongside the tests, positive controls must be carried out using samples in which ‘holes’ are made.
The use of samples rather than standard controls allows the test to be truly simulated. Compared with certain deterministic methods, the use of positive controls means that the detection limit on the real sample can be rechecked at each test session.
Note that detection limits vary according to the type of container. The larger the container (several litres, for example), the higher the detection limit. The detection limit is correlated to the capacity of the liquid to penetrate the container.
For example, for a 20-litre flexible liquid bag, the detection limit will generally be greater than 100μm, whereas for a syringe or acon of a few millilitres, the detection limit may be lowered to 30μm with sufficiently strong pressure variations.
To generate the positive controls, microtubes or microcapillaries can be used to simulate the micro-holes in the samples.
Creating positive controls is a delicate task. During preparation and then during insertion of the capillaries into the samples, it is important not to clog the capillaries or break them.
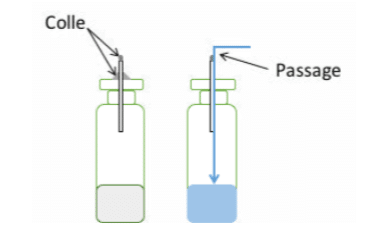
Another essential point is the fixing of the capillaries. If a capillary is not sealed or not properly sealed to the sample, the liquid will flow past it rather than into it (see Figure 1), even if the capillary is inserted into a rubber stopper.
The rubber stopper does not shrink perfectly around the capillary, leaving a passage for the liquid. It should be noted that there is also a correlation between the length of the capillaries and the probability of passage through them, hence the need to carry out validation before undertaking routine tests.
Advantages and disadvantages of this method
This method has a number of well-known disadvantages:
- This is a “destructive” method, and the samples must not be re-used,
- Directly linked to the previous point, the waste generated must be managed, and care must be taken with products soiled with methylene blue.
- Detection limits are relatively high, but can be improved by applying large variations in pressure,
- The analysis time is long: most of the time, the blue needs at least 30 minutes to penetrate.
Despite these drawbacks, this method is still advantageous in many cases:
- It works equally well on small and large volumes, and also on packaging of different shapes and sealed using different methods.
- It’s fairly simple to set up and doesn’t require expensive equipment.
[1] PDA, Development of a Dye Ingress Method to Assess Container-Closure Integrity: Correlation to Microbial Ingress
[2] USP: The U.S. Pharmacopeial Convention





